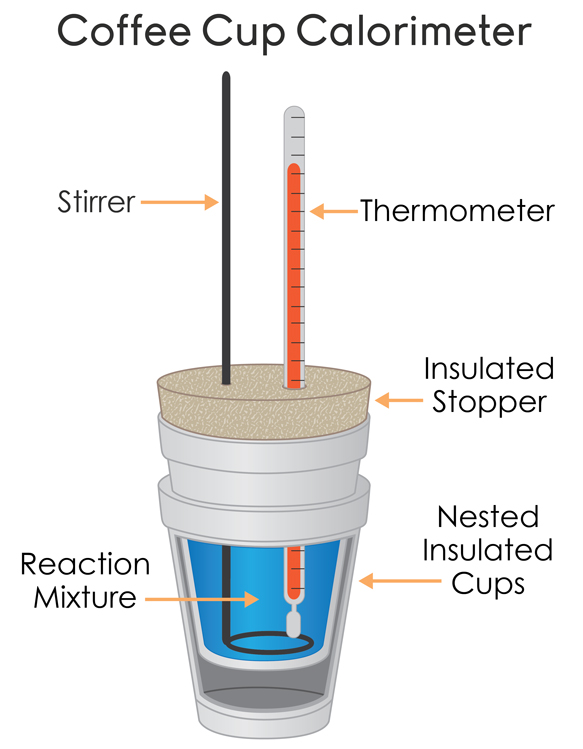
Calorimeter Constant Negative . apply the first law of thermodynamics to calorimetry. a negative δh means that heat flows from a system to its surroundings; what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A positive δh means that heat flows into a system from its surroundings. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. We need to find the difference between the heat lost by the hot water when it droped. there are two main types of calorimetry: in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. Compare heat flow from hot to cold objects in an.
from www.w3schools.blog
A positive δh means that heat flows into a system from its surroundings. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. what is the calorimeter constant? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We need to find the difference between the heat lost by the hot water when it droped. there are two main types of calorimetry: apply the first law of thermodynamics to calorimetry. a negative δh means that heat flows from a system to its surroundings; Compare heat flow from hot to cold objects in an. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry.
Cp, Cv calorimetry W3schools
Calorimeter Constant Negative We need to find the difference between the heat lost by the hot water when it droped. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. We need to find the difference between the heat lost by the hot water when it droped. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. apply the first law of thermodynamics to calorimetry. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. Compare heat flow from hot to cold objects in an. a negative δh means that heat flows from a system to its surroundings; A positive δh means that heat flows into a system from its surroundings. there are two main types of calorimetry: what is the calorimeter constant?
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Calorimeter Constant Negative what is the calorimeter constant? there are two main types of calorimetry: a negative δh means that heat flows from a system to its surroundings; in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. apply the first law of thermodynamics. Calorimeter Constant Negative.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Definition Chemistry Calorimeter Constant Negative in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. We need to find the difference between the heat lost by the hot water when it droped. apply the first law of thermodynamics to calorimetry. what is the calorimeter constant? A positive δh. Calorimeter Constant Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Calorimeter Constant Negative We need to find the difference between the heat lost by the hot water when it droped. there are two main types of calorimetry: A positive δh means that heat flows into a system from its surroundings. what is the calorimeter constant? apply the first law of thermodynamics to calorimetry. Constant pressure, or coffee cup calorimetry, and. Calorimeter Constant Negative.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant Negative there are two main types of calorimetry: Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. a negative δh means that heat flows from a system to its surroundings; A. Calorimeter Constant Negative.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Constant Negative apply the first law of thermodynamics to calorimetry. there are two main types of calorimetry: in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. Compare heat flow from hot to cold objects in an. a negative δh means that heat flows. Calorimeter Constant Negative.
From exoarnnpu.blob.core.windows.net
Calorimeter Description And Function at Kyla Ochs blog Calorimeter Constant Negative a negative δh means that heat flows from a system to its surroundings; Compare heat flow from hot to cold objects in an. Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. there are two main types of calorimetry: We need to find the difference between the heat lost by the hot water when it. Calorimeter Constant Negative.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant Negative there are two main types of calorimetry: in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. what is the calorimeter constant? Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. apply the first law of thermodynamics to calorimetry.. Calorimeter Constant Negative.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter Constant Negative a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A positive δh means that heat flows into a system from its surroundings. there are two main types of calorimetry: apply the first law of thermodynamics to calorimetry. in the specific situation described, qsubstance m is a. Calorimeter Constant Negative.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimeter Constant Negative We need to find the difference between the heat lost by the hot water when it droped. a negative δh means that heat flows from a system to its surroundings; A positive δh means that heat flows into a system from its surroundings. in the specific situation described, qsubstance m is a negative value and qsubstance w is. Calorimeter Constant Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Constant Negative apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an. what is the calorimeter constant? We need to find the difference between the heat lost by the hot water when it droped. there are two main types of calorimetry: Constant pressure, or coffee cup calorimetry, and constant volume, or. Calorimeter Constant Negative.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog Calorimeter Constant Negative Compare heat flow from hot to cold objects in an. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. what is the calorimeter. Calorimeter Constant Negative.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, Calorimeter Constant Negative Compare heat flow from hot to cold objects in an. a negative δh means that heat flows from a system to its surroundings; what is the calorimeter constant? A positive δh means that heat flows into a system from its surroundings. apply the first law of thermodynamics to calorimetry. We need to find the difference between the. Calorimeter Constant Negative.
From www.reddit.com
Calculating calorimeter constant is coming out negative and don’t know Calorimeter Constant Negative Compare heat flow from hot to cold objects in an. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred. Calorimeter Constant Negative.
From www.w3schools.blog
Cp, Cv calorimetry W3schools Calorimeter Constant Negative Constant pressure, or coffee cup calorimetry, and constant volume, or bomb calorimetry. Compare heat flow from hot to cold objects in an. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. a calorimeter is a device used to measure the amount of heat. Calorimeter Constant Negative.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimeter Constant Negative in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. apply the first law of thermodynamics to calorimetry. A positive δh means that heat flows into a system from its surroundings. there are two main types of calorimetry: Constant pressure, or coffee cup. Calorimeter Constant Negative.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Calorimeter Constant Negative Compare heat flow from hot to cold objects in an. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. We need to find the. Calorimeter Constant Negative.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant Negative there are two main types of calorimetry: in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. what is the calorimeter constant? Constant. Calorimeter Constant Negative.
From www.numerade.com
SOLVED Calorimetry Lab 1. In the measurement of a calorimeter Calorimeter Constant Negative what is the calorimeter constant? in the specific situation described, qsubstance m is a negative value and qsubstance w is positive, since heat is transferred from m to w. there are two main types of calorimetry: apply the first law of thermodynamics to calorimetry. A positive δh means that heat flows into a system from its. Calorimeter Constant Negative.